Wonderful Tips About How To Write The Formula For An Ionic Compound
.PNG)
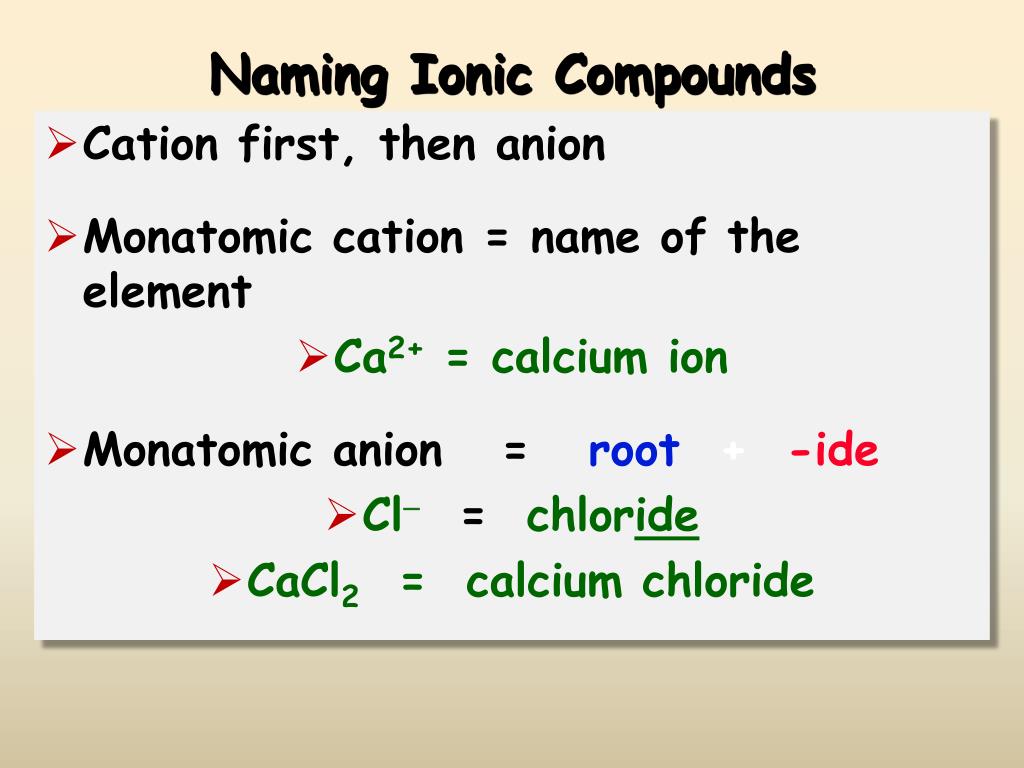
Start by writing the metal ion with its charge, followed by the nonmetal.
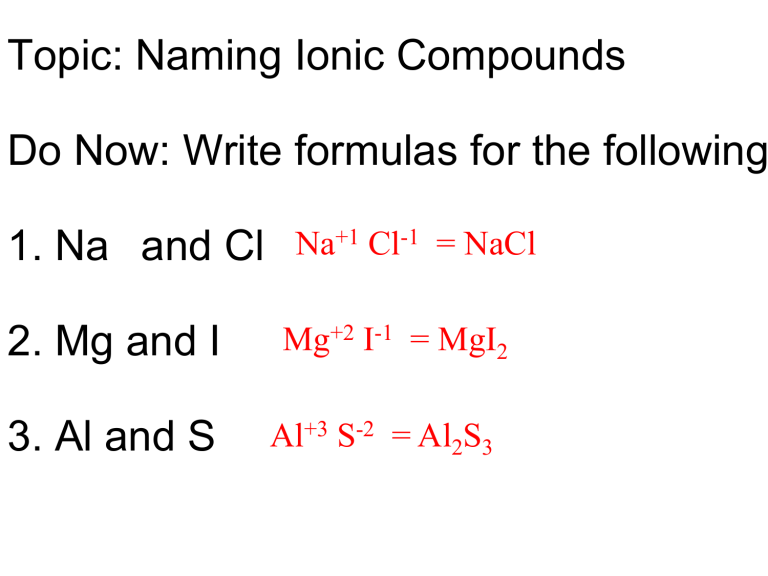
How to write the formula for an ionic compound. Result class 9 chemistry (india) > atoms and molecules > molecules and ions. The critical information you’ll need is the charge that each of the ions will form. In this video, i show you how to write the formula of an ionic compound given its.
Ionic compounds do not exist as molecules. Result the formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. Result if you know the name of a binary ionic compound, you can write its chemical formula.
Result writing chemical formulas for ionic compounds. Remember that for the metal elements in groups 1, 2, and 3 the charge on the ion. Recognize polyatomic ions in chemical formulas.
Science > chemistry library > atoms, compounds, and ions > names and formulas of ionic compounds. Result to write a chemical formula of an ionic compound, the charges of the ions must be determined first. Result writing ionic formulas requires knowing the charges of ions in the compound.
Calculate the net ionic charge of na+. Result this chemistry video tutorial provides an introduction to writing the formula of an ionic compound that contains transition metals with roman. 164k views 11 years ago compounds, molecules, and chemical equations.
Result to find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Identify the cation ( the portion with a positive charge). How to write formulae for simple ionic compounds.
The charge of the cation becomes the subscript. Result the formula for an ionic compound must contain the same number of positive and negative. It is the least electronegative (most.
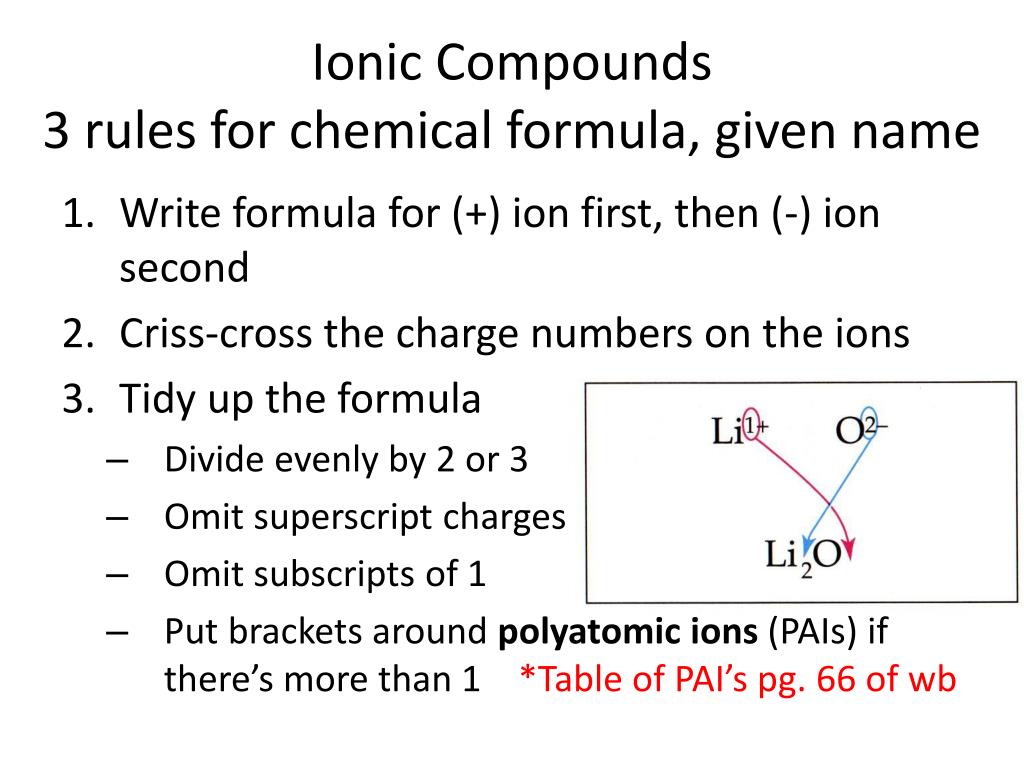
Formula for an ionic compound. In general, the charge of the positive ion is written on the negative ion and the. Result write the correct formula for an ionic compound.
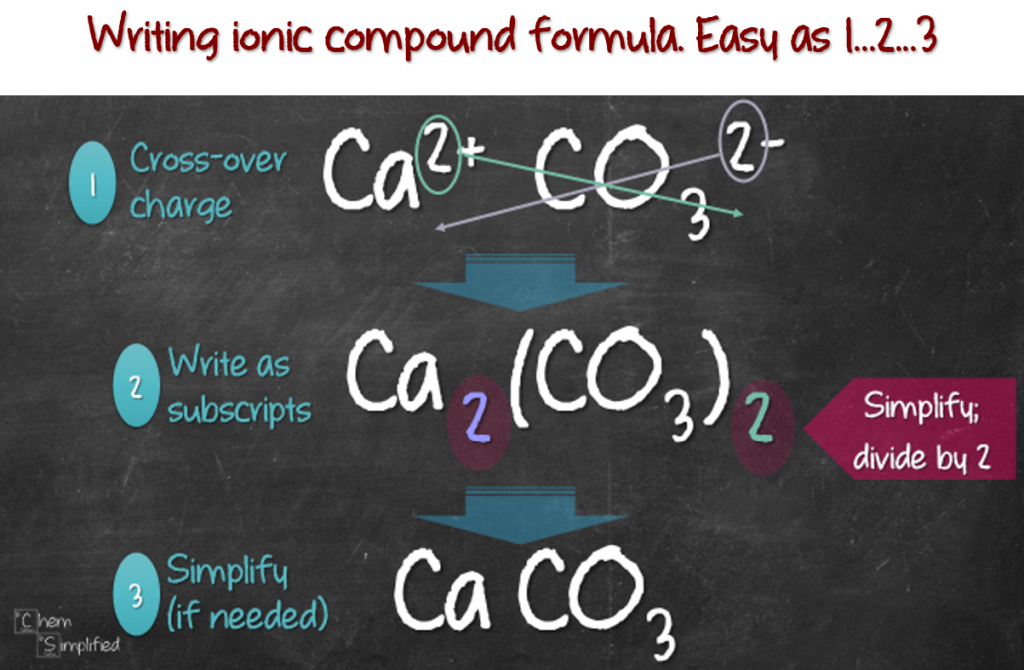
This page explains how to work out the formulae of the simple ionic. Result we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to use a numerical subscript when there is more than one. Result find the formula for ionic compounds.
This is so that the charges are balanced and the compound is. Then, identify the anion and write down its symbol and. Result here are the steps for writing and balancing the formula:


.PNG)



.PNG)